WWTF funded Project “Structure Zoom” (LS17-008)
Zooming in on protein functional sites with atomic resolution – an integrated chemistry approach for structural biology.
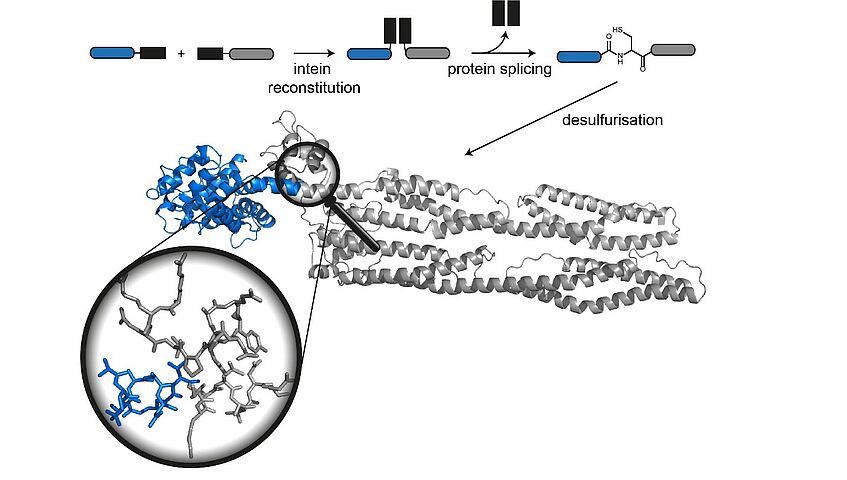
In the Structure Zoom project, we aim to apply chemical protein synthesis techniques to generate site-specifically labeled and modified proteins for structural biology of key proteins in neurodegeneration and muscle disease. Our unique interdisciplinary expertise in protein synthesis, site-specific modification and structural biology makes it feasible to design and synthesize protein constructs that provide precise information about structural features and dynamics by NMR and small angle scattering (SAS). The innovative aspects of the project lie in our ability to generate specifically isotope-labeled and/or posttranslationally modified proteins by combining synthesis of building blocks with protein synthesis. With this versatile approach, we can access protein samples that have so far not been available for NMR and SAS studies. As a proof-of-principle, we will apply our chemical toolbox of labeling and semisynthesis strategies to two proteins (tau-4 and FATZ) and use the resulting structural and dynamic data on the interactions of these proteins with their respective binding partners to provide insights into their molecular structural organization. Furthermore, this project establishes a new partnership between the peptide and protein chemistry group at the University of Vienna and the structural biology center at the Max F. Perutz Laboratories, enabling unique interdisciplinary training of young researchers at the interface of these two disciplines.
Participants:
Institute of Biological Chemistry, Faculty of Chemistry, University of Vienna
Christian F. W. Becker (Coordinator)
School of Biomedical Sciences, University of Queensland
Anne C. Conibear
Max F Perutz Laboratories, University of Vienna
Kristina Djinovic-Carugo, Robert Konrat
Institute of Organic Chemistry, Faculty of Chemistry, University of Vienna
Roman Lichtenecker
Publications:
Sponga, A., Arolas, J. L., Schwarz, T. C., Jeffries, C. M., Chamorro, A. R., Kostan, J., Ghisleni, A., Drepper, F., Polyansky, A., Ribeiro, E. D. A., Pedron, M., Zawadzka-Kazimierczuk, A., Mlynek, G., Peterbauer, T., Doto, P., Schreiner, C., Hollerl, E., Mateos, B., Geist, L., Faulkner, G., Kozminski, W., Svergun, D. J., Warscheid, B., Zagrovic, B., Gautel, M., Konrat, R., and Djinović-Carugo, K. (2021) Order from disorder in the sarcomere: FATZ forms a fuzzy but tight complex and phase-separated condensates with {alpha}-actinin. In press in Sci Adv
